The Big Picture
How a molecule responds when it is displaced from equilibrium by interaction with a photon or through collision, is fundamentally important problem that defines the chemistry of the Earth’s atmosphere and the interstellar medium, the generation of industrial plasmas and radiative damage to biological molecules. This molecular response can occur across a wide-range of timescales, including the ultrafast timescale of attoseconds to picoseconds. To study chemistry on this timescale in the lab, we use a combination of state-of-the-art ultrafast laser spectroscopy methods and custom-built mass spectrometers and ion/electron imaging apparatus to build-up a more complete picture of how an initial response to ionisation can dictate the final product outcomes.
In particular, we are focusing our efforts on making new tools to better understand the chemistry of molecular ions. These systems are not only ubiquitous and therefore important in their own right, but they are also appealing systems to try to understand the ultrafast dynamics of the ground electronic state. There has been over three decades of cutting-edge research in the field of femto-chemistry; work that has almost exclusively has addressed the chemistry of electronically excited molecules. However, the overwhelming majority of chemistry occurs on the electronic ground state. Our hope is, that by exploiting the relative simplicity of being able to prepare radical cations in internally excited levels of their electronic ground state, we can use this as a framework for making general predictions and testing theories of ground state chemistry on the ultrafast timescale.
This blue-sky research often finds application in unforeseen places, but at the most basic level we are trying to develop empirical validation of quantum mechanical models, and create new tools to interrogate and potentially manipulate chemical reactions in real-time. Our current projects are listed below, but we believe it is important for us to be able to follow interesting directions as they arise.
CURRENT PROJECTS
-
1) Ultrafast Dissociative Ionisation
Ionisation is a fundamental aspect of the chemistry of a wide range of environments. For example, radiation and particle beam therapies that are used to treat disease such as cancer will indisriminately ionise the tissue surrounding the target site. This processes can liberate secondary electrons that initiate further irreversible damage to critical biomolecules such as DNA, proteins or peptides.

Electron ionisation of DNA, migration of the hole along the backbone, and finally strand breakage.
Historically, the chemistry of ionised molecules has been rationalised by statistical mechanical theories that assume that the internal energy has been equilibrated throughout the molecule through intermolecular vibrational redistribution (IVR) prior to reaction. These theories fail to capture processes that outcompete IVR, so called non-ergodic dynamics. IVR is a picosecond process, and so we need experiments and theory that address the chemistry of these processes on their native, ultrafast timescale.
In the NURD Lab, we use tunnel ionisation driven inelastic electron rescattering with a femtosecond laser pulse to ionise and excite complex molecules in the gas phase. By using femtosecond pump-probe mass spectrometry and covariance analysis, we can follow the kinetics and dynamics of multiple reactions in a single measurement. By tracking the reaction from start-to-finish, we can learn about the connections between reactants, intermediates and products and develop a comprehensive understanding of the complex chemistry of ionised molecules.

a) Schematic of the time-of-flight mass spectrometer we use to study ultrafast pump-probe ion chemistry; b) schematic of the laser induced desorption technique used to prepare large molecules for investigation in the gas phase
-
2) Molecular Movies and Ground State Dynamics
To understand the transition-state of a chemical reaction is to understand the fundamental link between reactants and products. However, the transition-state is an ephemeral concept that can only be inferred from experimental results and theoretical models. A 'Molecular Movie' is almost exactly what it sounds like; it is an attempt to bypass inference and look directly at chemistry in motion by taking a series of images that when combined, show how the molecular structure evolved during the reaction.
Making a Molecular Movie is also about as challenging as it sounds. There are several approaches to doing it depending on the system at hand, and each technique relies on a slightly different set of experitise. So, we collaborate! Our group periodically join forces with other groups from around the world to visit state-of-the-art research facilities called X-ray Free Electron Lasers (XFEL) and use advanced charged-particle and/or diffractive imaging techniques to try to capture electronic and nuclear dynamics in real-time.
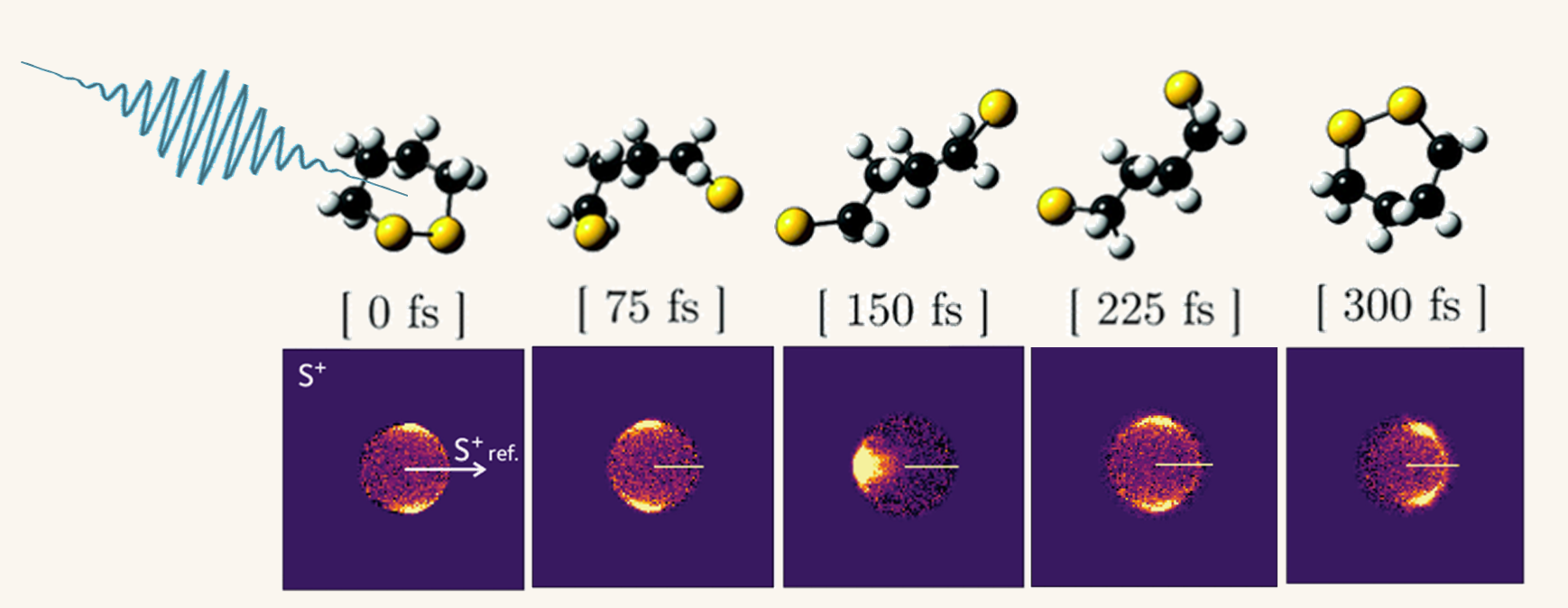
UV-induced structural dynamics of 1,2-dithiane. Top row: evolving molecular structure taken from quantum dynamics simulations, bottom row: Recoil frame covariance maps of sulfur ions recoiling from another sulfur ion travelling in the positive x-direction at the same time steps
Our favourite ultrafast structure determination technique is Coulomb explosion imaging (CEI). A Coulomb explosion occurs when a molecule is stripped of multiple electrons faster than the nuclei can move in response. This leaves behind a highly positively charged (and very unhappy) ion that 'explodes' due to the Coulombic force between the neighbouring positive charges. If you can reliably measure the momenta of several of these ions in coincidence or covariance, then it is possible to reconstruct the initial positions of each atom in the molecule at the moment of explosion. The figure above shows some recent work by our collaboration at the Artemis Facility at the Rutherford Appleton Laboratory, where we used CEI to watch the UV photochemistry of a cyclic disulfide.
In our lab at Nottingham, we are in the early stages of building a "Reaction Microscope" that is able to detect multiple ions and/or electrons in coincidence. This instrument will let us to perform CEI experiments on molecular ions prepared and probed using two intense femtosecond laser pulses. This work aimed towards being able to study how vibrationally energy accumulates in the active modes of reaction. This will allow us to better understand the energy transfer process, and more generally, the dynamics of 'thermally-activated' chemical reactions.

A diagramatic description of the working principle of the reaction microscope momentum imaging system
Collaborations are critical for the success of almost all of our work. We have a wide network of friends and collaborators who each make important contributions to our research, and we aim to offer the same in return. We are always happy to hear from people who might want to discuss the possibility of working together.
Currently, we work closely with Dr Matthew Robinson at the Small Quantum Systems Instrument at EuXFEL on structural dynamics of cyclic disulfides. We also have an experiment-theory collaboration with the Kirrander Group at the University of Oxford.